-

专业包装 正品保证
-

快乐服务 售后无忧
-

会员特权 优惠不断
-

个人信息 严格保护
| 别名: | IL7,Interleukin-7 | ||
|---|---|---|---|
| 储存条件: | -20℃ | ||

|
| 货号 | 规格 | 可用库存 | 销售价(RMB) | 您的折扣价(RMB) | 购买数量 |
|---|
| 熔点: | |
|---|---|
| 密度: | |
| 储存条件: | -20℃ |
GMP Human IL-7
IL7,Interleukin-7
GMP Human IL-7 is expressed from human 293 cells. It contains AA Asp 26 - His 177.
Predicted N-terminus: Asp 26
Request for sequence
This protein carries no "tag".
The protein has a calculated MW of 17.4 kDa. The protein migrates as 26-32 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
Less than 10 EU/mg by the LAL method.
<0.5 ng/μg of protein tested by ELISA.
<0.02 ng/μg of protein tested by DNA fluorescent staining method.
The sterility testing was performed by Membrane Filtration Method described in CP<1101>.
Negative
Negative
>95% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4. Normally trehalose is added as protectant before lyophilization.
Please see Certificate of Analysis for specific instructions.
Shipping at ambient temperature. Upon receipt, store it immediately at -20°C or lower for long term storage.
This product is stable after storage at:
-20°C to -70°C for 24 months in lyophilized state from date of receipt;
-70°C for 12 months under sterile conditions after reconstitution.
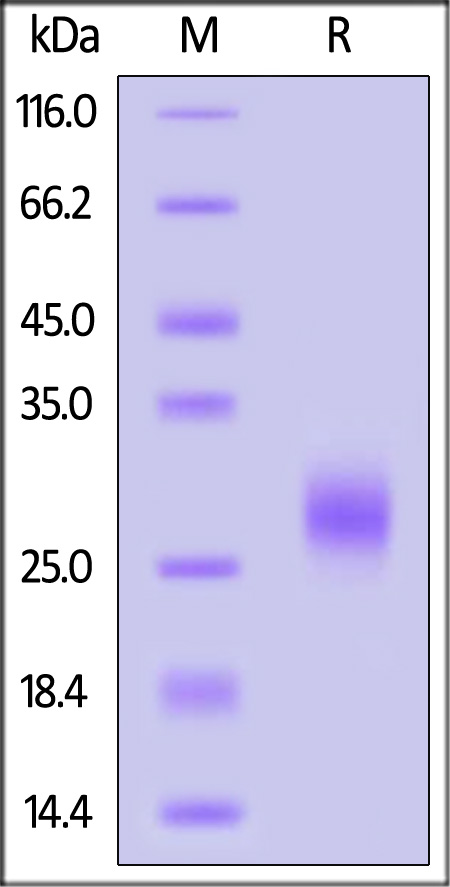
GMP Human IL-7 on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%.
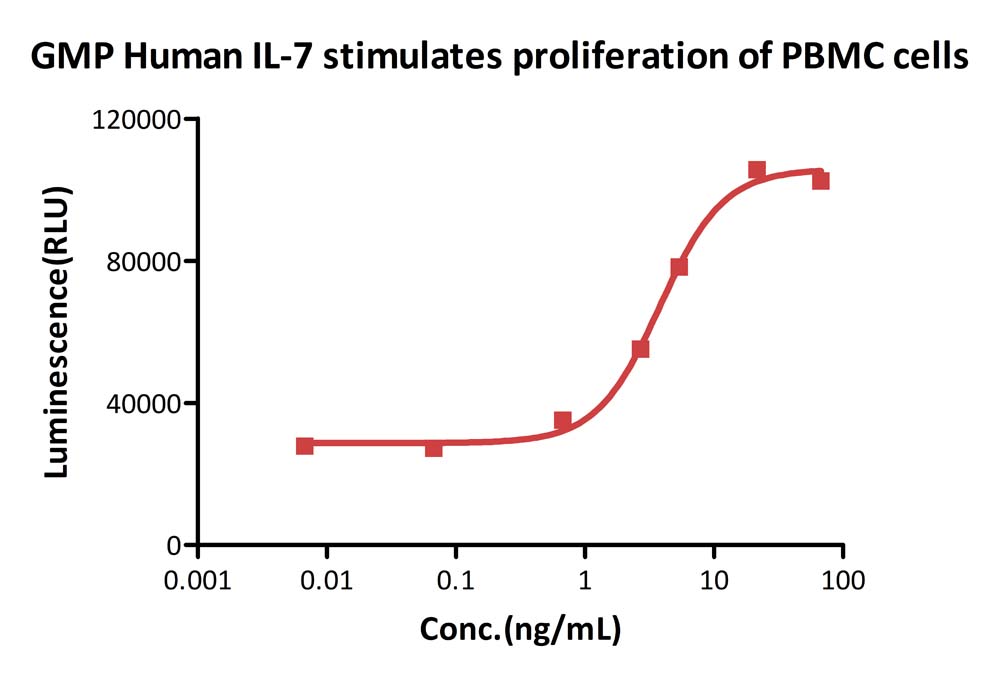
GMP Human IL-7stimulates proliferation of PHA-P-activated human peripheral blood mononuclear cell (PBMC). The EC50 for this effect is 3.821 ng/mL, corresponding to a specific activity of > 1.0 ⅹ10^8 IU/mg, which is calibrated against human IL-7 WHO International Standard(QC tested).
Protocol
ACROBiosystems GMP grade products are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP <92> Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems Quality Management System Contents:
Designed under ISO 9001:2015 and ISO 13485:2016, Manufactured and QC tested under a GMP compliance factory.
Chemically defined cell culture media, serum free and Xeno free, BSE/TSE free raw materials
Materials purchased from the approved suppliers by QA
ISO 5 clean rooms and automatic filling equipment
Qualified personnel
Quality-related documents review and approve by QA
Fully batch production and control records
Equipment maintenance and calibration
Validation of analytical procedures
Stability studies conducted
Comprehensive regulatory support files
Request For Regulatory Support Files(RSF)
ACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
SDS-PAGE
Protein content
Endotoxin level
Residual Host Cell DNA content
Residual Host Cell Protein content
Biological activity analysis (Reference the WHO Human IL-7 (NIBSC code: 90/530) as standard)
Microbial testing
Mycoplasma testing
In vitro virus assay
Residual moisture
Batch-to-batch consistency
ACROBiosystems GMP grade products are designed for research, manufacturing use or ex vivo use. CAUTION: Not intended for human in vivo applications.
All products are warranted to meet ACROBiosystems Inc.’s (“ACRO”) published specifications when used under normal laboratory conditions.
ACRO DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, ACRO DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOT WITH STANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN ACRO AND PURCHASER FOR THE PURCAHSE OF THE PRODUCTS, ACRO’S TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO ACRO FOR THE RELEVANT PRODUCTS. IN NO EVENT WILL ACRO BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
The End User is aware that ACROBiosystems Inc. and its affiliate (“ACRO”) sell GMP grade products designed for research, manufacturing use or ex vivo use and not intended for human in vivo applications. The End User further agrees, as a condition of the sales of ACRO’s GMP grade products that: a) the End User will not use this GMP grade product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the applicable review board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.